F.-Ulrich Hartl
Research Department Cellular Biochemistry
Molecular Chaperones, Protein Folding, Proteostasis, Aging and Neurodegenerative Diseases
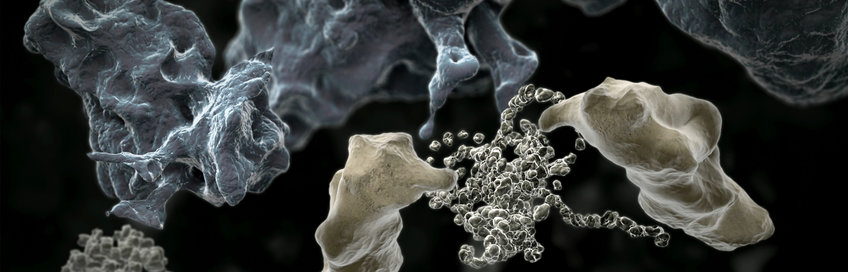
Many proteins have complex molecular architectures that do not form efficiently via a spontaneous folding process. Helper proteins, called molecular chaperones, ensure that such proteins fold into their correct shape and do not clump together to non-functional (or even toxic) aggregates in living cells. Molecular chaperones therefore play an important role in maintaining a functional proteome, also referred to as protein homeostasis or proteostasis. Scientists in the Department "Cellular Biochemistry" investigate the mechanisms of molecular chaperone functions in protein folding and related cellular processes. They have elucidated the function of an important group of chaperones, the chaperonins, which are barrel-shaped complexes that enclose an unfolded protein molecule and allow it to fold in a shielded environment unimpaired by aggregation. The department also studies the role of toxic protein aggregates in neurodegenerative diseases such as Alzheimer's, Parkinson’s and Huntington's disease. The aggregates form in an age-dependent manner and interfere with key cellular functions. Understanding these mechanisms will provide important information in developing novel therapeutic strategies for this group of presently incurable conditions.
News

Congratulations to Cole Sitron
for winning the main poster prize at the
ASAP Collaborative Meeting, London, October 2023!

Thanks to the Hartl-Fighters for a wonderful
MPIB-Sommerfest 2023
Group Leaders



