Protein folding helpers in their natural environment
Scientists from Martinsried and Göttingen have analyzed protein folding helpers, so-called chaperonin complexes, in their natural environment using cryo-electron tomography.
Cryo-electron tomography, or cryo-ET for short, can be used to visualize and analyze cellular structures in their natural environment. Researchers at the Max Planck Institute of Biochemistry (MPIB) in Martinsried near Munich and the University Medical Center Göttingen have now used cryo-ET to study protein folding helpers, so-called chaperonin complexes, in the bacterium Escherichia coli. These chaperonins help newly synthesized proteins to fold into their correct, functional form. The researchers were able to illuminate the folding reaction with unprecedented detail, monitoring conformational changes in the chaperonin as well as its interactions with the client protein inside the folding chambers. The results have been published in the journal Nature.
Research Briefing in Nature
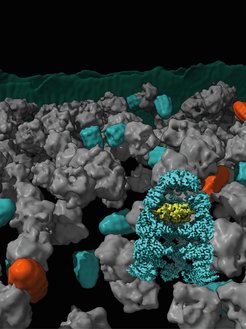
Protein Folding Helper Chaperonin
Proteins are the molecular building blocks of life. In order to perform a multiplicity of functions in the cell, each protein must adopt a defined three-dimensional structure, similar to components in machines. Protein folding assistants, called chaperonins, help newly synthesized proteins achieve their functional shape.
Biochemist F.-Ulrich Hartl, Director at the MPI of Biochemistry explains: "I have been working with chaperonins for more than 30 years. The protein complex exists unchanged in almost all living organisms and is essential for proper protein folding and cell survival. Misfolded proteins are associated with diseases such as Alzheimer's and Parkinson's. Understanding the structure and function of chaperonins may help us in developing new strategies for the treatment of these diseases.”
In order to gain a better understanding of how chaperonins work, Hartl collaborated with the structural biologists Wolfgang Baumeister, Emeritus Director and inventor of cryo-ET at MPIB, and Rubén Fernández-Busnadiego from the Institute of Neuropathology at the University Medical Center Göttingen (UMG) and member of the Cluster of Excellence “Multiscale Bioimaging: From Molecular Machines to Networks of Excitable Cells” (MBExC).
Chaperonin Complex
In bacteria, chaperonin complexes consist of two distinct subunits, GroEL and GroES. GroEL is organized into two stacked rings that form a barrel. GroES acts as a lid for the GroEL barrel. Newly produced proteins are encapsulated in the nanometer-sized barrel and allowed to fold while being protected from the cellular environment.
The researchers were able to show two main forms of the GroEL-GroES complex, known as "bullet" and "football" (named after the shape of an American football) within cells. The forms differ in their structural symmetry. In the bullet form, a GroES cap is bound to only one side of the GroEL barrel. This shape was found predominantly in bacteria under normal growth. In addition, football complexes were also detected. The microscopic images also showed that the proteins to be folded were located in the chaperonin barrel. Jonathan Wagner, first author of the study and scientist in Martinsried and Göttingen, explains: "It is fascinating that cryo-electron microscopy is now so advanced that we can follow process like protein folding in such detail in living cells".
Rubén Fernández-Busnadiego describes: “In this study, we combined cryo-ET with single-particle cryo-electron microscopy (cryo-EM) and quantitative mass spectrometry. This allowed us to observe different conformations of chaperonin complexes in different cellular states and determine their abundance. The ability to visualize these complexes directly in the bacterium instead of only in the test tube represents a major advance in this field and has only recently become possible, as this chaperonin complex is only 14 nanometer wide. Decades of experiments with purified GroEL/ES complexes led to conflicting results on how this machinery works. This is likely because in vitro experiments cannot fully recapitulate the conditions found inside the cell. We can now address this conundrum by cellular cryo-ET, as we can image the complexes at high resolution within native, unperturbed cellular environments.”
Ulrich Hartl concludes: “The results indicate that during chaperonin-assisted protein folding, the chaperonins assemble differently and alternate between the asymmetric ‘bullet’ and the symmetric ‘football’ shape in one reaction cycle. In future work, we will focus on elucidating the intermediate states of these cycles to understand how they are regulated by the chemical reactions of ATP binding and hydrolysis.”
Dictionary of the Research Department Cellular Biochemistry
ATP (adenosine triphosphate): is a mononucleotide that has bound energy-rich phosphate residues. ATP serves as the central energy currency in biological processes.
Chaperone: Is a family of proteins that help newly synthesized proteins to fold.
Chaperonins: are large, barrel-shaped protein complexes that enable correct, ATP-dependent protein folding.
GroEL: central subunit of the chaperonin complex in the bacterium Escherichia coli
GroES: “lid” subunit of the chaperonin complex in the bacterium Escherichia coli
kDa: Abbreviation for kilo-dalton; indicates the molecular mass of proteins. 1 dalton = 1.66018 x 10-27 kg. This corresponds to the twelfth part of the mass of the carbon isotope 12C
Cryo-electron microscopy: cryos in Greek: cold; biological samples (e.g. purified proteins or cells) are shock-frozen in liquid ethane to prevent the formation of water crystals, enabling preservation in close-to-native conditions. The samples can then be visualized at high resolution with the help of electron microscopy.
Cryo-electron tomography: This imaging method allows 3D imaging of cryo-preserved specimens such as cells. Its high resolution allows determining protein structures within intact cellular environments.












