A Boost for Photosynthesis

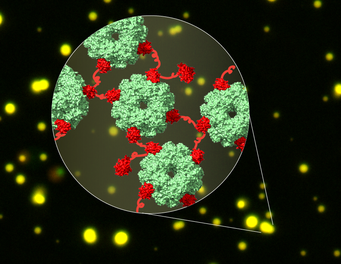
Cryo-EM structure of the linked complexes of CcmM (red) and Rubisco (green) in liquid droplets (yellow). Formation of this network is the first step in carboxysome biogenesis in cyanobacteria.
With the global population increasing by an estimated 80 million people each year, the demand for food is steadily on the rise. To meet this demand, scientists are working on strategies to improve the productivity of crops.
Photosynthesis – essential but inefficient
While most organisms have to take up nutrients to generate energy for their cells, plants and some microbes can fuel their cells with light energy. In a process called photosynthesis, they convert water and carbon dioxide (CO2) to sugar and oxygen with the help of sunlight. The critical enzyme of this reaction is Rubisco, which catalyzes the fixation of CO2 from the atmosphere. However, Rubisco, which is estimated to be the most abundant enzyme on Earth, works slowly and inefficiently. Instead of binding CO2, Rubisco can also react with oxygen. A lot of energy is lost in this side reaction. Therefore, scientists are trying to engineer a more efficient version of Rubisco to improve plant growth. A team led by Manajit Hayer-Hartl, head of the research group “Chaperonin-assisted protein folding” at the Max Planck Institute of Biochemistry in Martinsried, has now made an important contribution to these efforts.
Cyanobacteria are aquatic microbes that perform photosynthesis. They have evolved a strategy to increase the efficiency of their Rubisco enzyme. Cyanobacteria locally increase the concentration of CO2 in specialized structures called carboxysomes and confine their Rubisco to these structures. In this way, cyanobacteria reduce the energy that is lost in the side reaction with oxygen. The new study has now demonstrated how the formation of carboxysomes is initiated.
Trapping Rubisco for extra energy
Cyanobacteria use a helper protein called CcmM to capture Rubisco. CcmM is built of several repeat modules that resemble the small subunit of Rubisco – therefore it was long assumed that these modules replace the small subunits of Rubisco when the proteins interact, thereby linking Rubisco proteins. However, the complex between CcmM and Rubisco is highly dynamic and therefore could not be solved by traditional structural biology methods. “In this study, we took advantage of the rapidly developing method of cryo-electron microscopy to capture these dynamic interactions”, says Huping Wang, shared first author of the study. Using cryo-EM, the researchers showed that CcmM does not replace the small subunit of Rubisco but rather links Rubisco proteins together by an unexpected mechanism.
The interaction between CcmM and Rubisco causes de-mixing of the protein complex from other proteins in the cell. “This de-mixing of proteins is called phase separation, a process that concentrates proteins locally. In cyanobacteria, the protein shell of the carboxysome is then formed around the phase separated CcmM and Rubisco to capture them“, explains Xiao Yan, shared first author of the study. A similar process of phase separation also plays a role in neurodegenerative diseases such as Amyotrophic Lateral Sclerosis.
The formation of carboxysomes boosts photosynthesis because these microcompartments concentrate CO2 in the vicinity of Rubisco, making the enzyme more efficient. The current results greatly improve the understanding how these CO2-concentrating structures are formed. Manajit Hayer-Hartl describes a potential application of the results of the study: “If we could transfer functioning carboxysomes into higher plants, this would give crops an extra boost by allowing them to fix CO2 more efficiently. The energy, which is usually lost in the reaction of Rubisco with oxygen, would go towards the production of biomass “. In the long-term, the insights from Hayer-Hartl’s research could contribute to the generation of more efficient crops, reduce the demand for fertilizers and improve the global food supply. [CW]
Original publication:
Wang H*, Yan X*, Aigner H, Bracher A, Nguyen ND, Hee WY, Long BM, Price GD, Hartl FU, Hayer-Hartl M. Rubisco condensate formation by CcmM in β-carboxysome biogenesis. Nature, January 2019. *These authors contributed equally to this work.
https://www.nature.com/articles/s41586-019-0880-5